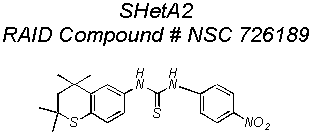
The most active Flex-Het, was submitted to the National Cancer
Institutes (NCIs) Rapid Access to Intervention Development (RAID) program
for funding of the Preclinical Testing needed to submit an Investigational New
Drug (IND) application to the Food and Drug Administration (FDA). The
Current and Completed Activity in the NCI RAID
Program:
Synthesis of
medium scale quantities (completed).
Formulation (oral
and perenteral)
Radioactive label
for research
Development of
HPLC-UV analytical method (completed).
Pharmacology
(partially completed).
Stability
(completed in plasma, but not formulation)
Extent of protein
binding in plasma (completed).
Metabolism
(partially completed)
Toxicology
dose-range finding study (In progress)
Efficacy (In
progress)
Ovary
Melanoma
Renal Cancer
Current Non-RAID Activity:
Clinical Trial
Design (OUHSC, GOG, Huntsman Cancer Institute)
Pending grants to
fund development of a pipeline of drugs to follow SHetA2 and to better
understand the mechanisms of SHetA2.
Next Phase of Activity Needed for
§
Scale up
synthesis and quality control for amounts of SHetA2 needed for clinical trials.
o
The
§
Funding for
clinical trials.