


| A 39 year-old Man with Primary Infertility. May, 2003, Case 305-3. Home Page |
Grant Davis, M.D. and Barbara L. Bane M.D. Last update: May 30, 2003.
Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
Clinical information: A 39 year-old man presented with a 12-year history of infertility. His previous spermiograms which demonstrated azoospermia. He had normal secondary male characteristics. His testosterone level was 386 ng/dL (reference range 270 - 1730 ng/dL), and his follicle stimulating hormone (FSH) level was elevated at 17.9 mIU/mL (reference range 1.0 - 7.9 mIU/mL). Past medical history was negative for mumps, testicular trauma, erectile dysfunction, chemotherapy, or hormone therapy. He had no known systemic illness. Physical examination revealed bilaterally descended testes that were slightly smaller than normal. No testicular masses were palpated. A testicular biopsy was performed and yielded two cores of soft cream-colored tissue of 0.4 cm in length. The specimen was fixed in Bouin's solution. Representative photomicrographs are shown below.
Pathology of the case:
 |
 |
 |
 |
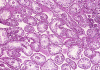
| A. | B. | C. | D. |
On
low-magnification (Panel
A), the
diameter of the seminiferous tubules appeared to be slightly decreased. There
is, however, no thickening of the basement membranes, interstitial fibrosis, or
inflammation. On medium and high- magnification
(Panel
B
and
C),the
seminiferous tubules are lined by columnar Sertoli cells which have triangular
to ovoid nuclei, pale-staining chromatin, and prominent central nucleoli. The
salient feature is the total lack of spermatogenesis
(Panel
C). The interstitium
contains a few scattered clusters of Leydig cells without definitive evidence of
hyperplasia (Panel
D).
| DIAGNOSIS: Sertoli cell-only syndrome (germinal cell aplasia), mature (adult) variant. |
Discussion: General Information Embryology Pathology
General Information
Primary testicular failure occurs in approximately 1% of all men, and is present
in 10% of those obtaining medical consultation for infertility.1
Sertoli cell-only syndrome (SCOS)
1,2,3, also known as germinal cell
aplasia, is not an uncommon finding in testicular biopsies that are performed in
these patients. The salient histopathologic feature of SCOS is the absence of
germ cells. The seminiferous tubules are lined by Sertoli cells which may
resemble immature (prepubertal) or mature Sertoli cells, or have other changes
which may correlate with specific etiologies and clinical findings. Many of
these patients are karyotypically normal and have normal secondary male sexual
characteristics, yet are infertile and azoospermic (having no detectable sperm
counts), or at most have very low sperm counts.
During the 1990s intracytoplasmic sperm injection (ICSI) was introduced, which
brought new relevance to the testicular biopsy, transforming it into a
therapeutic as well as a diagnostic procedure. With ICSI, doctors can retrieve
sperm from some men with SCOS, allowing them to have their own biological
children.4,5 Certain histopathologic features in testicular biopsies
in patients with SCOS have been found to correlate with successful sperm
retrieval procedures, principally the finding of focal clustered tubules
containing maturing germ cells (so-called mixed testicular atrophy).
To facilitate understanding of the classification of SCOS, a few words
concerning normal embryology, physiology, and histology of the testes are
included here. Germ cells originate in the yolk sac and migrate to the gonadal
ridge, and are later incorporated into the testes. Germ cell elements at various
stages of maturation comprise the majority of cells within normal adult
seminiferous tubules, outnumbering Sertoli cells approximately 13:1. Germ cells
undergo proliferation and renewal, with some maturing into spermatogonia.
Sertoli cells are essential in this process, both by forming the blood-testis
barrier and by producing a variety of substances essential for germ cell
maturation. Adult Sertoli cells have irregularly shaped (sometimes triangular)
nuclei, pale chromatin, and prominent nucleoli, unlike their immature
counterparts which have ovoid nuclei with a regular outline and inconspicuous
nucleoli. In normal adult tubules, Sertoli cells may be inconspicuous, obscured
by germ cells, but they are readily identified by their basal location and
prominent nucleolus. Adult seminiferous tubules average 180 mm
in diameter and have open tubular lumens.6
According to Nistal et al.,1,7,8 five morphologic variants of SCOC
are recognized, which include immature, dysgenetic, mature (adult-type), involuting, and
dedifferentiated. Recognition of these variants is important to assess the
etiology of germinal cell aplasia in a given patient. In addition, in a small
number of the patients with the mature and dysgenetic variants, focal
spermatogenesis may be observed. The dysgenetic, mature, and involuting variants
of SCOS are more commonly encountered than the immature and dedifferentiated
variants. The three former variants are associated with elevated follicle
stimulating hormone, normal or elevated luteinizing hormone, and normal
testosterone levels. This constellation of findings, taken together with Sertoli
cell-only histology, infertility, and azoospermia, was formerly known as the del
Castillo syndrome.
The immature variant is caused by a primary deficiency of FSH and LH
production that begins in childhood and, as a result, maturation and renewal of
germ cells does not occur. Diameters of the seminiferous tubule are generally
decreased in all forms of SCOS, but this is most prominent in the immature
variant, where tubular diameter may be less than 80.
Sertoli cells exhibit pseudostratification and are rounded or oval and have dark
chromatin. Tubular lumens are small or absent.
In the mature variant, the seminiferous tubules are lined by
mature-appearing columnar Sertoli cells, some which have roughly triangular
(so-called tripartite) nuclei and/or vacuolated cytoplasm. Seminiferous tubular
diameters are smaller than in normal adult testes, but larger than in immature
SCOS; tubular lumens are open. The putative pathogenesis is failure of migration
of germ cells from the primitive yolk sac to the gonadal ridge. In spite of
this, the Sertoli cells, under normal hormonal regulation, develop relatively
normally. Some patients with mature SCOS have a history of viral orchitis; many
cases are idiopathic.
The dysgenetic variant is characterized by Sertoli cells with some degree
of maturation, primarily of the cytoplasm. The pseudostratified nuclei do not
assume the tripartite configuration of mature Sertoli cells, but have irregular
outlines and sometimes have coarse chromatin granules. Sometimes an admixture of
mature and immature-appearing Sertoli cells is observed, with variation between
seminiferous tubules and even within tubules. Tubular lumens are generally
inconspicuous. Dysgenetic morphology has been associated with abnormalities of
the Y chromosome and cryptorchid testes.
The involuting variant of SCOS is characterized by atrophic changes of
the Sertoli cells; the nuclei are lobulated and have irregular outlines. Tubular
lumens are open and basement membranes are generally thickened. The interstitium
may be fibrotic. Presumably the cause of the atrophy in Sertoli cells is also
the cause of the loss of germinal cells; Leydig cells are variably involved.
Etiologies include irradiation, cancer chemotherapy, and cyclophosphamide.
Similar findings are observed in the normal aging process, and therefore some
cases may represent premature or accelerated aging.
In the dedifferentiated variant of SCOS, immature-appearing Sertoli cells
are present in otherwise normal seminiferous tubules. Similar to the immature
variant, the Sertoli cells have rounded nuclei and exhibit pseudostratification.
In contrast, however, the tubules are larger and have open lumens. Etiologies of
dedifferentiated SCOS include hormonal therapy for prostate cancer, cisplatin,
and estrogen given to transsexual patients. Fibrosis and thickening of the
basement membrane are not features of this variant.
Other authors have used different classifications that are not entirely possible
to be compared with that by Nistal et al., described above. Anniballo et al.1
divided SCOS into two categories: pure (congenital) and mixed
(secondary). The pure form in their conceptualization is caused by failure
of migration of germ cells. The mixed form is related to postnatal damage to
previously healthly testicular tissue. These authors state that retrieving germ
cells in cases of pure SCOS is impossible. Therefore, these cases should be
identified in order to spare unnecessary medical expenses and inconvenience to
patients. Positive immunostaining of seminiferous tubules for vimentin and
negative staining for cytokeratin was associated with pure SCOS. In mixed SCOS,
there are features that correlate with the focal presence of germ cells that may
be translated into increased likelihood of successful sperm retrieval are
present. These features include positive staining for lipids in Sertoli cell
cytoplasm that indicates of reabsorption of germ cells and presence of
telomerase activity. The combination of increased inhibin and normal serum FSH
levels is also an indication of the presence of spermatids.
Reference:
Anniballo
R, Ubaldi F, Cobellis L, Sorrentino M, Rienzi L, Greco E, Tesarik J. Criteria predicting the absence of spermatozoa in the Sertoli cell only
syndrome can be used to improve success rates of sperm retrieval. Human
Reproduction 2000;15:2269-2277.
Nistal
M and Paniagua R. Non-neoplastic diseases of the testis. In: Bostwick DG and
Eble JN. Urologic
Surgical Pathology. St. Louis: Mosby, 1997:498-501.
Levin
HS. Nonneoplastic diseases of the testis. In: Sternberg SS, Antonioli DA,
Carter D, Mills SE, Oberman HA, eds. Diagnostic
Surgical Pathology. 3rd Ed. Philadelphia:
Lippincott Williams & Wilkins, 1999:1952-3.
Schwarzer
JU, Fiedler K, v Hertwig I, Krusmann G, Wurfel W, Schleyer M, Muhlen B,
Pickl U, Lochner-Ernst D.
Sperm retrieval procedures and intracytoplasmic
spermatozoa injection with epididymal and testicular sperms. Urol
Int. 2003;70:119-23.
Seo
JT, Ko WJ.. Predictive factors of successful testicular sperm recovery in
non-obstructive azoospermia patients. Int J Androl. 2001;24:306-10.
Trainer
TD. Testis and Excretory Duct System. In: Sternberg SS, ed. Histology
for Pathologists. 2nd Ed. Philadelphia: Lippincott-Raven
Publishers,1997:1019-1028.
Nistal
M, Jimenez F, Paniagua R. Sertoli cell types in the Sertoli cell-only
syndrome: relationships between Sertoli cell morphology and aetiology. Histopathology.
1990;16:173-80.
Nistal
M, Paniagua R.
Testicular biopsy: contemporary interpretation. Urologic
Clinics of North America. 1999;26:555-93.