


| A 45 year-old Man with a
Sellar Mass. March, 2004, Case 403-1. Home Page |
Marcella Koch B.A. (MS-IV)1, Benjamin White, M.D. 2, Kar-Ming Fung, M.D., Ph.D. 3 Last updated February 30, 2004.
1 Fourth year medical student, Class of 2004, College of Medicine, University of Oklahoma, 2 Department of Neurosurgery, and 3 Department of Pathology University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
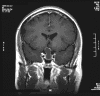
Clinical information: The patient was a 45 year-old man who presented with staring episode that is reminiscent of a seizure. MRI examination disclosed a cystic sellar mass that deflected his optic nerve. There was no clear visual loss.
 |
 |
 |
 |
 |
|
| A. | B. | C. | D. | E. |
Pathology of the case:
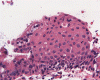
A dumbbell shaped cystic lesion with both sellar and suprasellar component is seen on T1-weighed image (Panel A). On low-magnification, part of the lesion is a cystic structure composed of a thin layer of supporting mesenchymal tissue and lined by a single layer of ciliated columnar epithelium (Panel B). The hemorrhage is probably resulted from the surgical procedure. The ciliated columnar epithelium with bland nuclei is well demonstrated in high-magnification (Panel C). In a small areas of the lesion, there are small clusters of squamous epithelium (Panel D). On high-magnification (Panel E), some of these squamous cell clusters are partially covered by ciliated columnar epithelium. These squamous cell clusters represent squamous metaplasia.
|
DIAGNOSIS: Rathke's cleft cyst |
Discussion: General Information Embryologic Surgical Imaging Pathology Differential diagnosis
General Information
Rathke’s cleft cyst is a benign cystic lesion that affects the mainly the pituitary gland and may have occasional recurrence. It is the most common incidental finding of the pituitary gland as per the study of an autopsy series. RCC comprise about 3.7% of all the incidental findings that are greater than 2 mm and they tend to be medially located 1. Our case is a good example for incidental finding as the patient is originally worked up for seizure. The subsequent MRI detected the incidental RCC that is not associated with visual symptoms.
Symptomatic Rathke’s cleft cyst (RCC), however, is relatively uncommon. When these cysts are large enough to be clinically apparent, they may cause headache, visual disturbances, nausea, decreased energy, vomiting, seizure, pituitary dysfunction, and diabetes insipidus, all due to mass effects 2, 3 on the pituitary stalk, the third ventricles and the surrounding structures. In one study, hemorrhage occasionally precipitates symptoms 15 . Of 27 RCC’s that were seen by CT or MRI and symptomatic in one series, all were above 3 mm in size 4. The age at which RCC’s become symptomatic varies among authors with a range from young adults to middle-aged patients but the mean age is 34 years-old as per one study 4. There is also a female predominance 4. Relief of symptoms is common with operative management with resolution of symptoms in 78% of cases in one series 2. The recurrence rate varies from 19-28% 2, 5.
During the third week of gestation, an evagination from the stomodeum (Rathke’s pouch) and an invagination of the floor of the third ventricle (the infundibulum) start to coalesce to form the pituitary gland 6. The portion that originates from the stomodeum requires sequential induction from the neighboring diencephalon to attain the morphology required to reach the infundibulum 7. Both of these entities are from ectoderm; Rathke’s pouch develops into the anterior and middle lobes, and the infundibulum develops into the posterior lobe and stalk of the pituitary gland. The lumen that connects Rathke’s pouch to the oral cavity begins being obliterated around the sixth week of gestation by the development of the sphenoid bone. The lumen of Rathke’s pouch is usually obliterated by proliferation of the anterior and posterior walls of the pouch that will become the anterior and middle lobes 6. The apical extremity of the Rathke's pouch will remain as a small remnant into postnatal life and adulthood as a small celft between the anterior pituitary (pars distalis) and the posterior pituitary (pars nervosa). The cleft is lined by columnar to cuboidal epithelium that are often ciliated and occasionally assocaited with mucous secreting globet cells. Accumulation of secretory products and form a cyst in this space. It should be noted that some authors have stated that one cannot differentiate a Rathke’s cleft cyst from a neuroepithelial cyst, such as a colloid cyst of the third ventricle, based on histology or histochemistry. They propose that some Rathke’s cleft cysts have neuroepithelial origin due to pinching off of cells from the infundibulum into the cells that make up the dorsal wall of Rathke’s pouch 8.
RCC has several distinctive features. They are most often intrasellar cyst with or without suprasellar extension. Entirely suprasellar RCC can also occur. Radiologically, RCC occurs as single, unilocated, well demarcated cyst without calcification. The content is homogenous. Depending on the content of the cyst, the MRI signal intensities can vary greatly. The radiological features overlap with that of craniopharyngiomas particularly those with extensive cystic changes and without calcification, cystic pituitary adenoma, and arachnoid cyst.
RCC are well-defined, small, thin walled cyst with a watery to mucinous content. Those with prior hemorhage may have an inspissated and brown content. The smaller RCCs usually occur as a small cyst at the interface of the anterior and posterior pituitary; most of these small cysts are asymptomatic. Histologically, RCCs are lined by a single layer of cuboidal to columnar cells that are often ciliated. Occasional mucous cells may be present. Squamous metaplasia may occur and raises the possibility of a craniopharyngioima. Occasional cells of anterior pituitary gland may be present. In RCCs that have substantial prior hemorrhage, there may be cholesterol cleft and foreign body giant cell reaction assocaited with old blood clot.
Differential diagnosis
RCC
should be distinguished from other lesions of the sellar/suprasellar region. Craniopharyngioma
is a relative common lesion in this area and also common in children and young
adults. On imaging grounds, crangiopharyngioma cannot always be satisfactorily
distinguished from RCC
3,
5.
In well-preserved tumor, the distinction between craniopharyngioma and RCC is
distinct. However, some craniopharyngioma and some RCC may have significant
hemorrhage that would attenuate the features of these features. Thorough
examination of the specimen is of critical importance. Attenuated minute
fragments of squamous cells admixed with hemosiderin laden macrophages and,
sometimes, cholesterol clefts may be present in these lesions. The presence of
ciliated columnar epithelium and the absence of tissue with genuine features of
craniopharyngioma favor a diagnosis of RCC. It should also be noted that
ciliated epithelium can also be found in crangiopharyngioima. The distinction is
important because craniopharyngioima is a locally aggressive and has a local
recurrence rate about three times higher than that of RCC
2,
5.
Both RCC and craniopharyngioima are believed to originate from Rathke’s cleft.
The overlapping pathology of RCC and craniopharyngioma suggest that they may
represent the two ends of a spectrum of lesions
13,
14.
Colloid cysts of the third ventricles
are seen in older patients (third to fifth decades of life). The location of
colloid cyst often makes it an effective block of foramen of Monro leading to
acute hydrocephalus. Its pedunculated nature, however, allows change in position
easily and release of the pressure built up. As a result, they are often
associated with intermittent headache. Sudden death has been well documented.
Smaller cysts may remain asymptomatic. Colloid cyst varies form several
millimeters to 3-4 cm. The cyst is round, unilocular and have a thin wall. After
fixation, the cyst content appears as a gray hyaline substance with consistency
of soft cartilage. Colloid cyst of the third ventfricle cannot be histologically
distinguished from Rathke’s cleft cyst. Imaging and clinical correlation is
important for the distinction.
Click
here to see differentiation of RCC from other cystic lesions
Histologic distinction of cystic pituitary adenoma, arachnoid cyst and RCC is usually obvious.
Reference:
Teramoto
A, Hirakawa K, Sanno N, Osamura Y.
Incidental Pituitary Lesions in 1,000 Unselected Autopsy Specimens.
Radiology.
1994; 193: 161-164.
Kasperbauer
JL, Orvidas LJ, Atkinson JL, Abboud CF.
Rathke Cleft Cyst:
Diagnostic and Therapeutic Considerations.
Laryngoscope.
2002; 112:1836-1839.
Mukherjee JJ, Islam N, Kaltsas G, Lowe DG, Charlesworth M, Afshar F, Trainer PJ, Monson JP, Besser GM, Grossman AB. Clinical, Radiological and Pathological Features of Patients with Rathke’s Cleft Cysts: Tumors That May Recur. J C Endo Metab. 1997; 82: 2357-2362.
Ross DA, Norman D, Wilson CB. Radiologic Characteristics and Results of Surgical Management of Rathke’s Cysts in 43 Patients. Neurosurgery. 1992; 30: 173-179.
Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic Lesions of the Pituitary: Clinicopathological Features Distinguishing Craniopharyngioma, Rathke’s Cleft Cyst, and Arachnoid Cyst. J C Endo Metab. 1999; 84: 3972-3982.
Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalons. Development. 1998; 125: 4835-4840.
Shuangshoti
S, Netsky MG, Nashold BS Jr.
Epithelial Cysts Related to Sella Turcica.
Arch Path.
1970; 90: 444-450.
Liu
JK, Weiss MH, Couldwell WT.
Surgical Approaches to pituitary tumors.
Neurosurg Clin N Am.
2003; 14: 93-107.
Koren
I, Hadar T, Rappaport ZH, Yaniv E.
Endoscopic Transnasal Transsphenoidal microsurgery Versus Sublabial Approach
for the Treatment of Pituitary Tumors: Endonasal Complications.
Laryngoscope.
1999; 109: 1838-1840.
Alfieri
A, Schettino R, Tarfani A, Bonzi O, Rossi GA, Monolo L.
Endoscopic Removal of an Intra-Suprasellar Rathke’s Cleft Cyst: Case
Report and Surgical Considerations.
Minim Invas Neurosurg.
2002; 45: 47-51.
Day JD. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin N Am. 2003; 14: 109-122.
Yoshida
J, Kobayashi T, Kageyama N, Kanzaki M.
Symptomatic Rathke's cleft cyst. Morphological study with light and electron
microscopy and tissue culture. J Neurosurg.
1977; 47: 451-458.
Harrison MJ, Morgello S, Post KD. Epithelial cystic lesions of the selar and parasellar region: a continuum of ectodermal derivatives? J Neurosurg. 1994; 80: 1018-1025.