



MGT
ATPase
ATPase
PAS
ORO
NADH-TR
NADH-TR
Merosin
Dystrophin
Desmin

Desmin
Semithin
Jiang Qian, M.D., Ph.D.1,
Uma Alumpur M.D.2,
Kar-Ming Fung, M.D., Ph.D.3
Last update
June 30, 2005.
A 76 year-old Man with
Weakness in Arms and Hands.
October, 2004, Case 410-1.
Home Page
1 Department of Pathology, Albany Medical College, Albany, NY, 2 Kingston Neurological Associates, Kingston, NY, and 3 Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
Clinical information: The patient was a 76 year old right-handed black male who experienced progressive weakness in both arms and hands for the past few years. According to the patient, his shoulders became weak at first. He also had difficulty in getting up from a seating position, deviation on flexion of his fingers, and numbness of his hands when they were cold. There was no pain in his back, arms and legs. He denied any numbness or tingling of his legs or bowel/bladder incontinence. He had intermittent constipation and experienced recent changes in bowel habits. There was no history of trauma or falls. He underwent shoulder surgery about 18 months ago but the symptoms of his shoulder did not improve.
His past medical history included hypertension and increased cholesterol level. He used to smoke cigars. For the past 20 some years he had 2-3 glasses of wine each night, but stopped in about 9 months ago. He was married for 40 years with no children, widowed in about 18 years ago and lived alone. The patient was also diagnosed for depression in the past.
Physical examination revealed weight loss, weakness of arms, and stiffness of joints. He showed no cognitive deficiency and had normal cerebellar function. Muscles in the arm and hands were atrophic. He had significant weakness of upper extremities and the muscle strength is 2/5 in deltoid, 3/5 in biceps and triceps, and 3/5 in brachioradialis, 3/5 in wrist flexion and extension. He had ulnar palsy in hands for over 10 years with the left side more affected than the right. Significant weakness in the muscle of the hands was also noted. There was no pain in the shoulders on palpation. Muscle strength in lower extremities was 5/5 in all muscles except for 4/5 in iliopsoas. The patient could squat up and down without much difficulty. Sensory functions were intact in proprioception and light touch. There was bilateral decrease in pinprick below the knees. Reflexes were trace in upper extremities and were 2/4 knee jerk and 1/4 ankle jerk. There were no pathologic reflexes. The functions of cranial nerve II to XII were intact. There was good, function in the sternocleidomastoid muscles, but atrophic latissimus dorsi, trapezius, infraspinatus and supraspinatus muscle. He had percussion tenderness of his bilateral ulnar cubital tunnels and right median tunnel. Cervical and lumbar spines had full range of movement and were nontender. CT scan of C-spine showed spondylosis in C3-7. Head CT showed mild brain atrophy. Electromyogram and nerve conduction studies of upper extremities were reported as suggestive of motor axonopathy. Laboratory studies demonstrated slight elebation of rheumatoid factor to16.3, the sedimentation rate was 13, creatine kinase in serum was 171, and Lyme’s test was negative. A muscle biopsy was perform as part of his workup. Representative photos from the muscle biopsy are shown below:
All stains and histochemistries are performed on frozen materials.
 |
 |
 |
 |
 |
 |
| A. | B. |
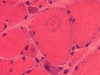
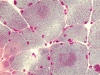
C. MGT |
D. ATPase |
E. ATPase |
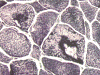
F. PAS |
 |
 |
 |
 |
 |
 |
|
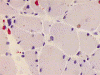
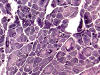
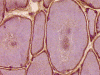
G. ORO |
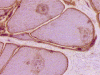
H. NADH-TR |
I. NADH-TR |
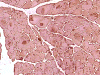
J. Merosin |
K. Dystrophin |
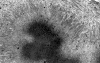
L. Desmin |
 |
 |
 |
 |
 |
 |
|
M. Desmin |
N. Semithin |
O. | P. | Q. | R. |
| HE | Hematoxylin-eosin stain. | PAS | Periodic acid Schiff reaction. |
| MGT | Modified Gomori's trichrome stain. | Merosin | Immunohistochemistry for laminin-2 (merosin) |
| ATPase | ATPase reaction at pH 9.4. | Dystrophin | Immunohistochemistry for dystrophin |
| PAS | Periodic acid Schiff reaction. | Desmin | Immunohistochemistry for desmin. |
| ORO | Oil red O. | Semithin | 1 mm resin section, toulidine blue stain. |
| NADH-TR | NADH-tetrazolium reductase reaction. | EM | Electron microscopy, magnification in numbers (X1000). |
Pathology of the case:
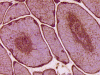
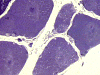
On hematoxylin-eosin stained sections, there is an increased variation of fiber diameter with many atrophic fibers intermingled with fibers of normal caliber. There is no evidence of fiber grouping or perifascicular atrophy. No inflammatory cells are present. There is also mild interstitial fibrosis (Panel A). On higher magnification, many fibers have a round concentric structure (Panel B). Irregular, centrally located depositions are also identified on modified Gomori's trichrome. The concentric nature, however, is not as obvious as in the hematoxin-eosin stained sections (Panel C). Type I and II fibers are not clearly separated in the ATPase preparation at pH 9.4. This is a common situation in chronically ill muscle (Panel D). The type I fibers are unusually dark. There is an increase in the proportion of type I fibers. The atrophic fibers are usually type II fibers. The concentric lesions are found predominantly in type I fibers (Panel E). There is an increase in PAS staining which is consistent with increased glycogen storage (Panel F). No increase in lipid content is demonstrated by oil red O (Panel G). On NADH-TR reaction, the concentric structures appear to have a clear central core that is devoid of enzymatic activity, a rim with intense enzymatic activity and a surround zone with relatively normal reactivity. These features are classic for target fibers (Panel H and I). No deficiency of laminin-2 (merosin) (Panel J) or dystrophin (Panel K) is demonstrated by immunohistochemistry. The central lesions are also immunoreactive for both laminin-2 and dystrophin. Immunohistochemistry for desmin demonstrate a core of strong immunoreactivity and also strong reaction in the sarcoplasmic membrane (Panel L and M). The target structures are also well demonstrated on semithin sections (Panel N). On electron microscopy, z-disc streaming is a common finding and they are often admixed with a substantial amount of dense granular electron dense substance (Panel O and P). There are also numerous cytoplasmic bodies characterized by radiating intermediate filaments (spheroid bodies) (Panel Q and R).
| DIAGNOSIS: Desmin related myopathy (desminopathy). |
Discussion: General Information Pathology Molecular
General Information
Disturbance of myofibrillary and internal cytoskeletal proteins is the shared characteristic of a family of myopathies. Some of these myotpathies such as central core disease, multiminicore disease, and nemaline myopathies have been known for many years. Other members of this family including actinopathies, plecin deficiency, telethonin deficiency, myotilinopathy, myosin heavy chain depletion disease, autosomal dominant myosin heavy chain IIa myopathy, and desmin-related myopathies (DRM) are recently described entities. Some of these entities also fall into the category of protein surplus myopathies that is characterized by an abnormal accumulation of proteins 1.
DRM are a group of myopathies with the shared feature of abnormal accumulation of desmin, a 53 kD, type III intermediate filament that is specific to muscle fibers 2, 3. Formation of various cytoplasmic inclusion or storage is the shared histological feature. Desmin plays important roles in both skeletal and cardiac muscles. Intermediate filaments are intracellular fibrous proteins between 8 to 10 nm in diameter which is “intermediate” in diameter in between the thin and thick filaments in muscle cells and are grouped into 5 types. In skeletal muscle, desmin is detected under the sarcolemma, at the periphery of Z-discs and at the myotendinous and neuromuscular junctions. The subsarcolemmal actin cytoskeleton is linked to the z-discs through desmin and plectin, an intermediate filament linking protein. Desmin is also associated with synemin and paranemin in the mature muscle fibers. In developing muscle fibers, desmin is associated with vimentin and nestin. In essence, Desmin play a role in formation of the cytoskeleton network.
DRM has been known for many other names including desminopathy, desmin myopathy, desmin storage myopathy, spheroid body myopathy, cytoplasmic body myopathy, Mallory body myopathy, myopathy with intrasarcoplasmic accumulation of dense granulofilamentous material, and myofibrillar myopathy associated with desmin accumulation.
The peak age of onset is between 25-45 years of age but infants and older adults can also be affected. Severity can be highly variable. Clinically, it usually starts as distal muscle weakness with extension to proximal weakness in some severe cases. Proximal weakness manifesting in the form of facial, pharyngeal, fascioscapulohumeral weakness or respiratory muscle weakness, and even smooth muscle have been described. Muscular atrophy and cardiac problems are frequent features. The patients may have arrhythmias, restrictive or hypertrophic cardiomyopathy. Cardiac involvement may precede muslce weakness for years. Neurogenic atrophy is an uncommon finding. The serum creatine kinase is usually slightly elevated. Electromyogrphic studies usually show myogenic changes but mixed myogenic and neurogenic changes are also observed.
Molecular Genetics and Etiology
The etiology of DRM is heterogeneous despite similar histological changes. Both autosomal dominant and autosomal recessive transmission has been described in genetically transmitted cases. In autosomal recessive cases, homozygous mutations would lead to earlier onset of disease. Desmin is a highly conserved protein. Human desmin is encoded by a single copy of 8.4 kb gene on chromosome 2q35 4, 5. Both missense mutations and deletions have been described in desmin gene. The desmin gene is a relatively large and complex gene 6, 7, 8, 9, 10. The type and location of mutations may be translated into different clinical severity and outcome. In some cases of DRM, an R120G missense mutation has also been identified in the a-B crystalline gene 11, 12 which is a 20 kD cytoplasmic small heat shock protein that probably provides protective function for the intermediate filament network against stress-induced damages. In this type of cases, desmin deposition may be a secondary event. Familial cardiac and skeletal myopathy associated with excessive desmin deposition or DRM can therefore be produced by mutations in desmin gene and a-B crystallin gene.
The two major pathologic findings are inclusion bodies and granulofilamentous material. Type I fibers are predominantly affected. Inclusions are eosinophilic on hematoxylin-eosin stain and bluish on modified Gomori’s trichrome stain. These inclusions are often negative for oxidative enzymes. A strong reactivity for desmin can be demonstrated by immunohistochemistry. The abnormal accumulation can occur as cytoplasmic inclusions, subsarcolemmal inclusions, spheroid bodies (10-20 mm oval or spherical inclusions), and patches or “hyaline structures”. In some cases, there is Z-disc streaming but no masses are demonstrated by trichrome stain. Numerous negative areas may be demonstrated by oxidative enzyme reactions. An accentuated intermyofibrillar network is seen by antidesmin antibody staining. Under the electron microscope, spheroid bodies appear as cytoplasmic bodies with a dense, granular core surrounded by fine filaments in a coronary arrangement. Although they are conspicuous on light microscopy, they may not be as distinctive at the ultrastructural level.
Granulofilamentous materials, also known as dappled dense structures” are dense anatomosing and trabeculated granular material that is about 100 nm in diameter is accumulated in between myofibrils or under the sarcolemma. The Z-disc streaming may appear contiguous to the granular deposits. They also appear as “non-hyaline” structure on immunofluroesent microscopy.Cytoplasmic bodies characterized by radiating intermediate filaments (spheroid bodies) are also common.
A wide variety of proteins including a-B crystallin, other cytoskeletal proteins, b-amyloid, gelsolin and others have been detected as co-dep[ositions. Cardiac pathology is similar to that of the skeletal muscle. Abnormalities in smooth muscle in the gastrointestinal tract have also been described.
In the peripheral nerves, there may be enlarged myelinated fibers filled with neurofilaments. Non-myelinated fibers and Schwann cells do not have increase in intermediate filament; a feature that distinguish them from giant axon neuropathy 14.
Reference:
Goebel HH, Warlo I. Gene-related protein surplus myopathies. Mol Genet Metab. 2000 Sep-Oct;71(1-2):267-75.
Goebel HH, Goldfarb L. Desmin-related myopathies. In Structure and Molecular Basis of Skeletal Muscle Diseases. By George Karpati. Allen Press, 2002. Page 70-73.
Goebel HH, Warlo I. Gene-related protein surplus myopathies.
Mol Genet Metab. 2000 Sep-Oct;71(1-2):267-75.
Viegas-Pequignot E, Li ZL, Dutrillaux B, Apiou F, Paulin D. Assignment of human desmin gene to band 2q35 by nonradioactive in situ hybridization. Hum Genet. 1989 Aug;83(1):33-6.
Li ZL, Lilienbaum A, Butler-Browne G, Paulin D. Human desmin-coding gene: complete nucleotide sequence, characterization and regulation of expression during myogenesis and development. Gene. 1989 May 30;78(2):243-54.
Dalakas MC, Park KY, Semino-Mora C, Lee HS, Sivakumar K, Goldfarb LG. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N Engl J Med. 2000 Mar 16;342(11):770-80.
Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle
JW, Semino-Mora C, Sivakumar K, Dalakas MC. Missense mutations in desmin
associated with familial cardiac and skeletal myopathy.
Nat Genet. 1998 Aug;19(4):402-3.
Munoz-Marmol AM, Strasser G, Isamat M, Coulombe PA, Yang Y, Roca X, Vela E, Mate JL, Coll J, Fernandez-Figueras MT, Navas-Palacios JJ, Ariza A, Fuchs E. A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc Natl Acad Sci U S A. 1998 Sep 15;95(19):11312-7.
Park KY, Dalakas MC, Goebel HH, Ferrans VJ, Semino-Mora C, Litvak S, Takeda K, Goldfarb LG. Desmin splice variants causing cardiac and skeletal myopathy. J Med Genet. 2000 Nov;37(11):851-7.
Sjoberg G, Saavedra-Matiz CA, Rosen DR, Wijsman EM, Borg K, Horowitz SH, Sejersen T. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum Mol Genet. 1999 Nov;8(12):2191-8.
Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998 Sep;20(1):92-5.
Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999 May 25;96(11):6137-42.
Perng MD, Cairns L, van den IJssel P, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999 Jul;112 ( Pt 13):2099-112.
Sabatelli M, Bertini E, Ricci E, Salviati G, Magi S, Papacci M, Tonali P. Peripheral neuropathy with giant axons and cardiomyopathy associated with desmin type intermediate filaments in skeletal muscle. J Neurol Sci. 1992 May;109(1):1-10.