DEVELOPMENT OF SYNTHETIC RETINOIDS
The development of retinoids for prevention and treatment of cancer is part of a state-wide NIH-funded research project involving collaborators at Oklahoma State University (OSU). K. Darrell Berlin, Ph.D. is a Regents Professor in the Chemistry Department at OSU who synthesizes reduced-toxicity retinoids called heteroarotinoids. Eldon C. Nelson, Ph.D. is a Professor in the Department of Biochemistry at OSU who evaluates the metabolism of these heteroarotinoids. Dr. Benbrook has taken over the role of Principal Investigator on this project and her research group at OUHSC is responsible for screening the heteroarotinoids for anticancer activity and for identifying the molecular mechanism of action. At the molecular level, these compounds act by binding to six different nuclear receptors that act as transcription factors by binding to specific DNA sequence elements in the promoters of genes. By understanding which receptors and genes are regulated by the most active compounds, Dr. Benbrook’s group plans to identify the chemical structural features associated with anticancer activity. This information can then be used to design highly specialized retinoids to prevent or reverse tumorigenesis.
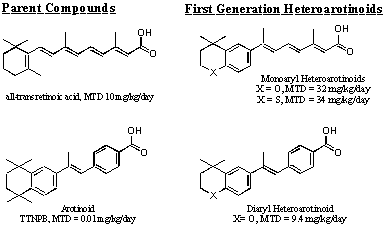
| The structures on the left show that the toxicity of the parent retinoid compounds is reduced by insertion of a heteroarotom (X) in the aryl ring to generate a heteroarotinoid. The MTD is the maximum tolerated dose determine by the dose that causes 10% weight loss after 60 days of administration in mice |  |
A series of heteroarotinoids were screened for inhibition of growth and activation of retinoic acid receptors in tumor cells. The biologic activity and low toxicity of these compounds is reported in Journal of Medicinal Chemistry 40:3567-3583, 1997 and in Gynecologic Oncology, 65(3): 425-429, 1997. The ability of heteroarotinoids to inhibit Head and Neck xenograft tumor establishment in mice is reported in Journal of Medicinal Chemistry, in press. This report also demonstrates strong retinoic acid receptor pan agonist activity by a sulfur heteroarotinoid and specific RXR retinoic acid receptor activity by an oxygen heteroarotinoid.
The most active compounds were tested for their ability to activate the six retinoic acid receptors. The effects of individual structural alterations on receptor specificity is reported in Journal of Medicinal Chemistry 41:3753-3757, 1998. A class of heteroarotinoids that contain a nitrogen atom were developed and described in Journal of Medicinal Chemistry, in press. Molecular modeling and biological assays demonstrated that a single methyl substitution could confer the capacity to activate the RARg retinoic acid receptor on a retinoid structure otherwise devoid of this activity. This RARg activation was shown to confer a strong activity against vulvar carcinoma cells. Although rare, vulvar cancer has a extremely high rate of recurrence after surgery and therefore, this a chemoprevention strategy that includes retinoid treatment after surgery would be of great benefit.
The most recent results include development of a retinoid inverse agonist that exhibits a different pharmacologic profile than conventional retinoids, manuscript in preparation.